Gynogenesis: an overview
Plants, like animals, have both female (ovules) and male (pollen) reproductive cells. Normally, the ovule needs to be fertilized by pollen to produce an embryo that will give rise to a new plant. However, did you know that it is possible to obtain embryos from only female reproductive cells? This phenomenon is called gynogenesis and it has been of wide interest for plant scientists to study plant mutations, to identify specific genes, to develop hybrid varieties and more.
If you want to learn more about this method, keep on reading and by the end of this article you will have a clear overview about the principles of gynogenesis.
What is gynogenesis?
In sexual reproduction, the union of male and female reproductive cells, also known as gametes, results in the formation of an embryo. This embryo contains the genetic information of both parents (diploid) and will give rise to a new plant. However, each gamete has the genetic information necessary to form the embryo independent of fertilization.
Gynogenesis is a process that leads to plants that exclusively originate from the female genetic background. That is, plantlets are developed without contribution of the male gamete. In a flower, the female reproductive part is the pistil, where ovules are contained in the ovary.
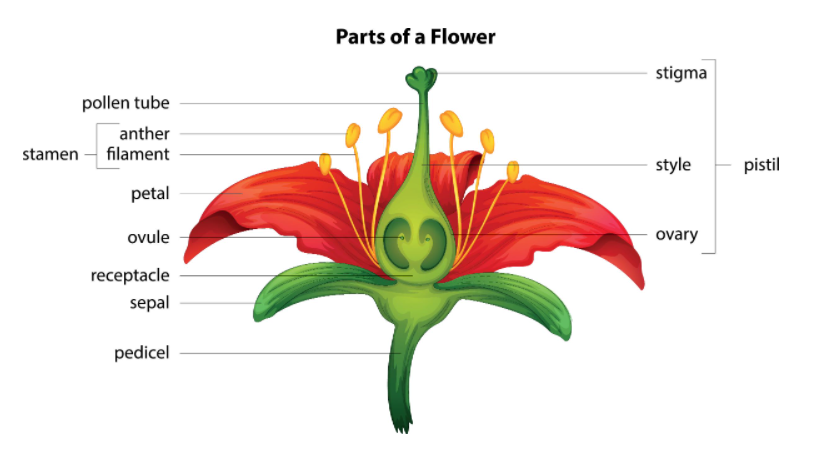
When you culture unfertilized ovules, ovaries, or flower buds to generate whole plants, it is called in vitro gynogenesis. It was first reported in 1964 by Tulecke in Ginkgo biloba. However, it was not until 1967 that it caught the attention of the plant science world, thanks to Noeum’s work in barley. Since then, plant production by gynogenesis has been reported in over 25 plant species!
Gynogenesis is the most important method of producing plants from only one parent (haploid plants) in species where androgenesis is not applicable. Androgenesis refers to the production of haploid plants from the culture of male gametes. You can read more about androgenesis and haploid plants in our article “Anther culture method-an overview”.
Did you know gynogenesis can occur naturally a well? In some plant species, like tobacco, wheat and apple, embryogenesis occurs spontaneously without pollen fertilization. This phenomenon is called parthenogenesis and is what we try to simulate in vitro.
Surprisingly, this is not unique to plants. Parthenogenesis occurs in some animals and even in humans! But, don't be so surprised, until now there has never been a case of babies born as a result of it.
How to produce gynogenic plants?
Plants produced through gynogenesis are called gynogenic haploids. These haploid plants are of great interest to plant breeders. Why is that? Because they help to reduce the time needed in the process of crop improvement. Now, you may wonder, how to produce them? Gynogenic haploid regeneration may happen by direct and indirect embryogenesis. During direct embryogenesis, the ovule directly develops into an embryo under in vitro conditions to grow plantlets. When the ovule is induced to form callus that later will differentiate into a whole plant, this is called indirect embryogenesis.
Efficient gynogenesis protocols generating a large number of embryos have been described. In some cases, you can pre-culture flower buds to isolate ovule/ovaries or use “sterile” pollen to stimulate embryogenesis. However, there is no universal protocol that is successful in all species. The basic steps involved in gynogenesis protocols are:
- Growth of donor plant;
- Development stage of ovary/ovule;
- Pretreatment;
- Embryo or callus induction;
- Regenerated embryo; and
- Haploid plants procurement.
There may be an additional step if you are looking to obtain fertile gynogenic plants. Normally, haploid plants are infertile. You can obtain fertile gynogenic plants through a process of chromosome doubling. This process is called double haploid technology and is useful in breeding because it allows the study of interesting characteristics through generations.
Also, you should consider that the efficiency of in vitro gynogenesis is influenced by plant-related factors and culture conditions. Some of the most important factors are:
- Genotype (refers to the genetic composition of the donor plant);
- Pre-treatment factors (such as heat and cold shock);
- Explant source and development stage (in species like onion, flower bud culture gives better results than ovule culture); and
- Culture medium (specifically, carbohydrate source and growth regulators).
Applications of gynogenesis
In practice, production of haploid plants by gynogenesis is not used as frequently as androgenesis in crop improvement programs because:
- The dissection of unfertilized ovaries and ovules is quite difficult; and
- The presence of small numbers of ovaries per flower compared to the large number of pollen grains in an anther.
Now, if there is androgenesis which is highly successful, why should you use gynogenesis? Gynogenesis is mainly used to produce haploids of male-sterile plants or of dioecious plant species (each individual has only female or male flowers), such as mulberry and cannabis.
The advantage this method offers you over androgenesis is the low occurrence of albinos (white plants without chlorophyll) in cereals like wheat, maize and rice. In crop plants like onion, sugar beet, and melon, gynogenesis is the only way to produce haploid plants. Also, in some plant species such as rice, gynogenesis is more efficient than androgenesis.
Onion case
Onion (Allium cepa L.) is a valuable crop for food and medicinal purposes. Therefore, through traditional methods and androgenesis, plant breeders have tried to produce new hybrids without much success. So, gynogenesis is the response! This method is useful for:
- Production of double haploid plants;
- Complex trait evaluation associated with onion quality, productivity, and resistance to environmental stress;
- Genetic mapping and identification of the genetic basis for traits like bulb color and restoration of fertility;
- Shorter breeding programs compared to traditional methods;
- Obtaining hybrids of commercial interest.
The future of gynogenesis may be even more promising with improved and refined methods. We hope this article was useful for you to understand this technique!
By Valeria Franco Franklin | 29-November-2021
About the author
Valeria Franco is from Colombia, the land of orchids. She is a focused and passionate biologist who specializes in biotechnology and molecular biology. Valeria has prior laboratory and research experience. She is presently employed as a content creator at Lab Associates and is always looking for new challenges. Valeria is enthusiastic in plant science themes and reading as a tool for lifelong learning. Her hobbies include studying foreign languages, traveling, and archery.
References
- Khan, P. S. S. V., Vijayalakshmi, G., Raja, M. M., Naik, M. L., Germanà, M. A., & Terry, R. G. (2020). Doubled haploid production in onion (Allium cepa L.): from gynogenesis to chromosome doubling. Plant Cell, Tissue and Organ Culture (PCTOC). doi:10.1007/s11240-020-01831-4
- Asif, M. (2013). Gynogenesis: An Important Tool for Plant Breeders. SpringerBriefs in Plant Science, 45–51. doi:10.1007/978-3-319-00732-8_3
- Bhojwani, S. S., & Dantu, P. K. (2013). Gynogenesis. Plant Tissue Culture: An Introductory Text, 113–118. doi:10.1007/978-81-322-1026-9_9
- Lentini, Z., González, A., Tabares, E., Buitrago, M. E., & Wêdzony, M. (2020). Studies on Gynogenesis Induction in Cassava (Manihot esculenta Crantz) Unpollinated Ovule Culture. Frontiers in Plant Science, 11: 365. doi:10.3389/fpls.2020.00365
- Chen, JF., Cui, L., Malik, A.A. & Mbira, K. G. (2011). In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tiss Organ Cult, 104:311–319. https://doi.org/10.1007/s11240-010-9874-6
- Ferrie, A.M.R. (2017).Doubled Haploid Production in Higher Plants. Editor(s): Brian Thomas, Brian G Murray, Denis J. Murphy. Encyclopedia of Applied Plant Sciences (Second Edition). Academic Press, 147-151. https://doi.org/10.1016/B978-0-12-394807-6.00189-1.
- Portemer, V., Renne, C., Guillebaux, A., & Mercier, R. (2015).Large genetic screens for gynogenesis and androgenesis haploid inducers in Arabidopsis thaliana failed to identify mutants. Frontiers in Plant Science, 6: 147. doi:10.3389/fpls.2015.00147
